Leading the way in de-risking clinical development with Al
De-risk clinical development and business decisions with AI
Extended timelines for drug approval of 10-15 years.

An ever-increasing average price tag to develop a drug, now at $2.68.

Low approval ratings, with only 12% of new molecular entities that enter clinical trials receiving FDA approval.



Our robust AI-driven suite of solutions empowers life sciences companies to de-risk high-stakes decisions. Our products enhance life sciences companies to assess the probability of success of a drug based on billions of expertly curated data points to make the process fast, highly consistent, unbiased and comprehensive.
We are raising the bar when it comes bringing groundbreaking therapies to market that will transform lives. Join us on this journey.
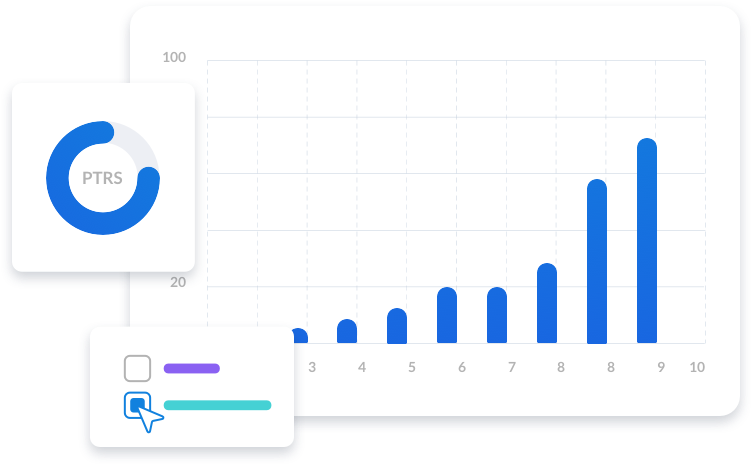
The patented SaaS platform Intelligencia Portfolio Optimizer™ delivers on-demand access to AI-powered insights based on best-in-class, proprietary data. This allows you to assess the probability of success (PoS) and phase transition probabilities objectively. Equipped with this knowledge, your pharmaceutical company can confidently make more informed asset, portfolio and licensing decisions.
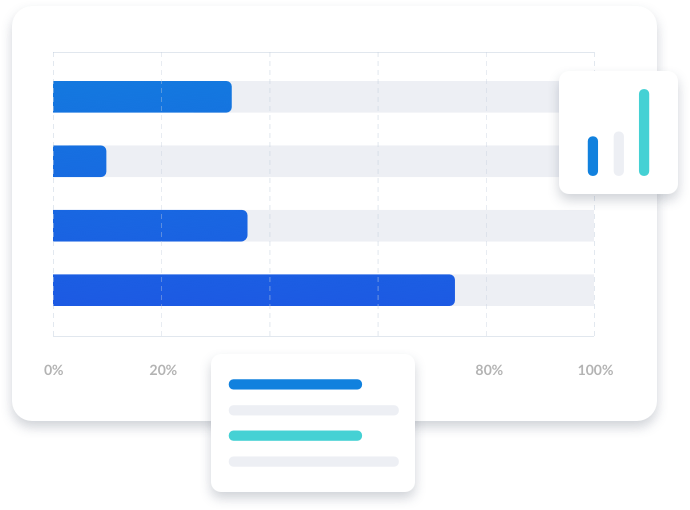
Focus on the specific indication and phase opportunity that matters to you versus high-level therapeutic area information. With access to our industry-leading, comprehensive historical approval and failure rates, augment your current risk assessment and enhance strategy to pave the way for future success.
Leverage meticulously curated and harmonized data for your bespoke and customized analyses needs. Collaborate with us as a trusted partner to augment your internal resources.



Focusing on portfolio planning, business development and licensing strategy, project management for long-range planning. We have you covered!

© Intelligencia AI. All rights reserved.